David Geier's Vaccine Research Review: Concerns And Controversy At HHS

Table of Contents
Dr. Geier's Research and its Focus
Dr. David Geier, an orthopedic surgeon, gained notoriety for his research questioning the safety of certain vaccines, particularly the MMR (measles, mumps, rubella) vaccine and the role of the preservative thimerosal. His work focused on potential links between vaccines and various adverse events, including autism spectrum disorder. While he initially published several papers on this topic, it's important to note that his research methodology and conclusions have been heavily scrutinized.
- Key publications and their findings: Dr. Geier's publications often highlighted correlations between vaccine administration and reported adverse events, although these correlations did not establish causation. Many were published in journals with lower impact factors or limited peer review.
- Methodology employed in his studies: Critics have pointed to methodological flaws in Dr. Geier's studies, including concerns about selection bias, small sample sizes, and a lack of robust controls. These weaknesses significantly impact the reliability and generalizability of his findings.
- Specific concerns raised by Dr. Geier regarding vaccine safety: His primary concerns centered on the potential long-term effects of MMR vaccination and the neurodevelopmental effects of thimerosal, a mercury-containing preservative that was largely removed from childhood vaccines in the United States. He argued for more thorough investigation into these potential links. This fueled the ongoing "thimerosal controversy."
Keywords: MMR vaccine safety, thimerosal controversy, vaccine adverse events, Geier research methodology.
The HHS Response and Investigation
The HHS, through its various agencies, responded to the concerns raised by Dr. Geier's research. This response involved investigations into the research methods and the potential for research misconduct.
- Timeline of events: The investigations spanned several years, involving reviews of Dr. Geier's publications and his research practices. Key milestones included the identification of specific methodological flaws and, ultimately, sanctions against Dr. Geier.
- Specific criticisms leveled by the HHS against Dr. Geier's work: The HHS criticized his research for lacking rigorous methodology, including issues with statistical analysis, flawed study designs, and insufficient peer review. The HHS concluded that his findings did not support his claims of causal links between vaccines and adverse health outcomes.
- Sanctions or actions taken against Dr. Geier: Dr. Geier faced significant consequences, including the retraction of some of his publications and restrictions on his ability to conduct further research. This highlights the importance of adhering to high standards of scientific integrity in vaccine safety research.
Keywords: HHS investigation Geier, vaccine research misconduct, scientific integrity, retraction of publications.
The Scientific Community's Response and Debate
The response from the broader scientific community to Dr. Geier's research and the subsequent HHS investigation has been overwhelmingly critical.
- Positions taken by leading medical organizations (e.g., CDC, WHO): The CDC and WHO, along with numerous other respected medical organizations, maintain a strong consensus on the safety and efficacy of vaccines. They have consistently rejected Dr. Geier's conclusions, citing the lack of robust evidence supporting his claims.
- Support for or criticism of Dr. Geier's work from other researchers: While some individuals have expressed support for his work, the vast majority of the scientific community rejects his conclusions due to the identified flaws in his research methodology. The overwhelming scientific consensus supports the safety of vaccines as currently administered.
- Citations of relevant peer-reviewed articles supporting both sides of the debate: A comprehensive search of reputable databases will reveal the significant imbalance in peer-reviewed publications supporting the safety and efficacy of vaccines versus those questioning their safety based on flawed methodologies.
Keywords: peer review process, scientific consensus on vaccines, vaccine hesitancy, public health debate.
Ethical and Legal Implications
The controversy surrounding David Geier's vaccine research raises significant ethical and legal implications.
- The impact of vaccine hesitancy on herd immunity: The spread of misinformation regarding vaccine safety, even stemming from flawed research, can contribute to vaccine hesitancy, undermining herd immunity and increasing the risk of vaccine-preventable diseases.
- Legal challenges related to Dr. Geier's research or the HHS's actions: There have been legal challenges surrounding aspects of this controversy, highlighting the complexities of navigating scientific disputes with significant public health implications.
- Ethical considerations regarding scientific integrity and the dissemination of research findings: This case underscores the vital importance of rigorous scientific methodology, transparency, and adherence to ethical guidelines in conducting and disseminating research, particularly in areas with significant public health consequences.
Keywords: vaccine hesitancy impact, public health implications, research ethics, legal challenges vaccine safety.
Conclusion
The controversy surrounding David Geier's vaccine research and the HHS's response highlights the complexities of vaccine safety discussions. While concerns about potential adverse effects are legitimate and warrant investigation, it is crucial to rely on rigorous, peer-reviewed scientific evidence. The overwhelming scientific consensus affirms the safety and efficacy of vaccines, and the flaws in Dr. Geier's research methodology have been widely documented. Understanding David Geier's vaccine research requires careful consideration of both sides, emphasizing the critical importance of evidence-based information. To learn more, search for reputable information using keywords like "evidence-based vaccine information," "CDC vaccine recommendations," or "WHO vaccine safety data." A critical review of David Geier's vaccine research underscores the need for transparency and rigorous scientific investigation in this vital area of public health.

Featured Posts
-
 Controversial Hhs Decision Anti Vaxxer To Examine Disproven Autism Vaccine Claims
Apr 27, 2025
Controversial Hhs Decision Anti Vaxxer To Examine Disproven Autism Vaccine Claims
Apr 27, 2025 -
 Brazil Bound Justin Herbert And The Chargers 2025 Season Opener
Apr 27, 2025
Brazil Bound Justin Herbert And The Chargers 2025 Season Opener
Apr 27, 2025 -
 Nbc Chicago Hhs Taps Anti Vaccine Advocate To Investigate Debunked Autism Vaccine Claims
Apr 27, 2025
Nbc Chicago Hhs Taps Anti Vaccine Advocate To Investigate Debunked Autism Vaccine Claims
Apr 27, 2025 -
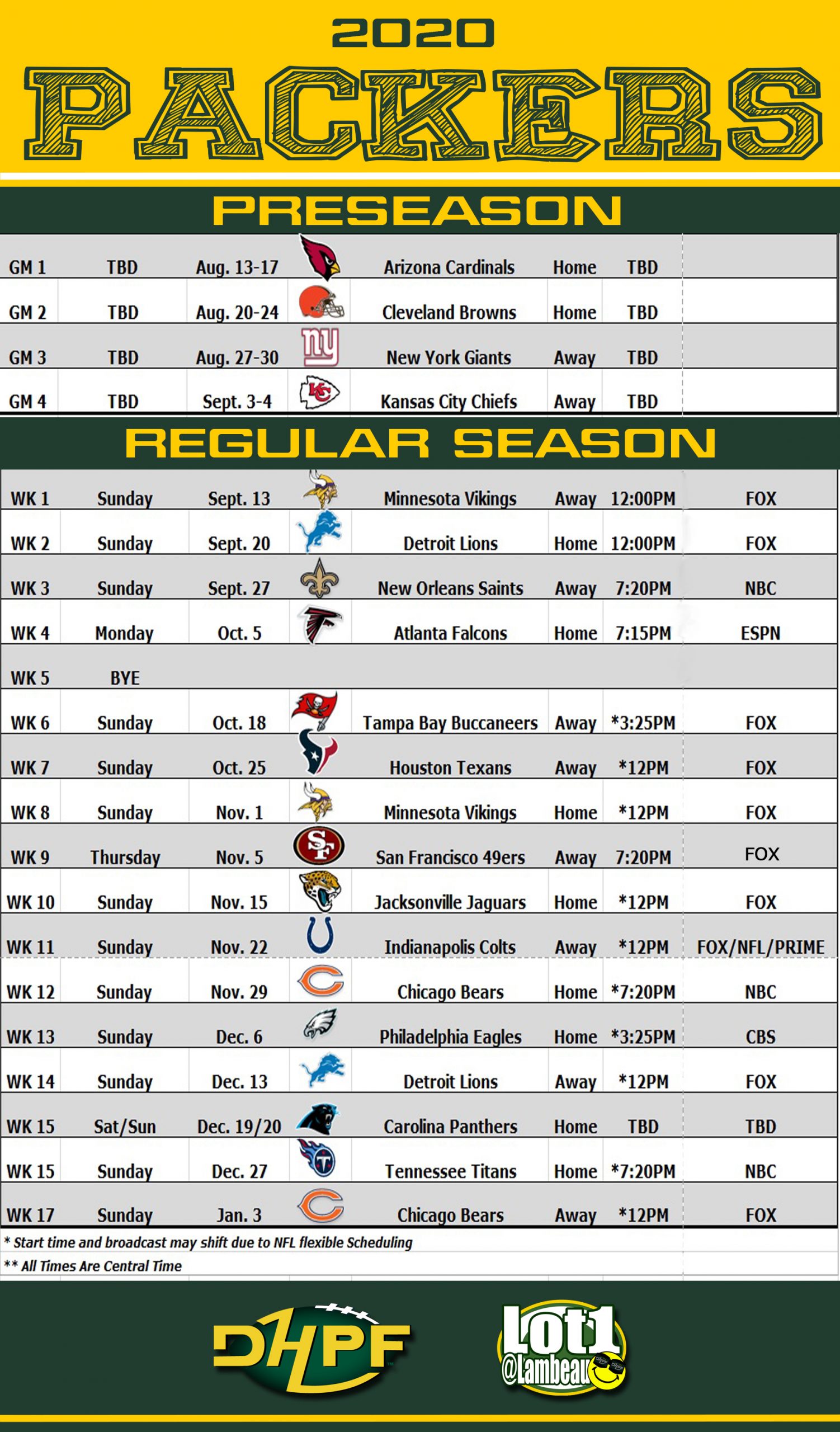 Nfl International Series 2025 Green Bay Packers Participation
Apr 27, 2025
Nfl International Series 2025 Green Bay Packers Participation
Apr 27, 2025 -
 Dealers Double Down Fighting Back Against Ev Mandates
Apr 27, 2025
Dealers Double Down Fighting Back Against Ev Mandates
Apr 27, 2025
